
Potassium thiocyanate is toxic by ingestion and emits a toxic gas if strongly heated.

Cobalt chloride complex skin#
Iron (III) nitrate solution may be a skin and body tissue irritant. These systems (detailed in Figure 1) are traditionally studied as part of an investigation of Le Châtelier’s Principle.įe 3+ (aq) is yellow, SCN - (aq) is colorless, and FeSCN 2+ (aq) is red-orange.Īdd iron (III) nitrate and potassium thiocyanate and heat/cool the solution. There are many different systems that illustrate this effect, including iron (III) thiocyanate and cobalt chloride complex, among others. The qualitative observations of the color changes allow students to predict and interpret various shifts in equilibrium systems as the rates of the forward and reverse reactions change until equilibrium is reestablished. Le Châtelier’s Principle is a favorite topic of my students because of the dramatic colors involved in the reactions. Traditional Le Châtelier’s Principle experiments The first green replacement lab that I implemented in my classroom was Beyond Benign's Equilibrium/Le Chatelier’s Principle lab. These greener replacement labs teach the same skills and address the same standards as traditional experiments, but utilize methods and chemicals that don’t expose our students to unnecessary risk, and are safer for the environment. Green chemistry’s approach to reducing risk - through the elimination of hazards and toxic chemicals - is more effective than trying to lower exposure through the use of PPE.Ĭlassroom and laboratory safety can be improved by reducing the use of hazardous materials by replacing traditional chemistry labs with greener alternatives. Risk, or the probability of a harmful event, is a function of both exposure and hazard, and green chemistry plays a role in improving laboratory safety by addressing the hazard component of the function. However, accidents and unintended outcomes can still happen in the laboratory, especially with inexperienced high school chemistry students. Protocols, procedures, and rules are implemented to make sure the laboratory is a safe place to learn and to reduce the incidence and severity of accidents.

Cobalt chloride complex how to#
Traditionally, this involves reducing the exposure to hazardous substances by showing students how to correctly use safety equipment and ensuring the use of proper personal protective equipment (PPE). This Flinn handout also gives important disposal instructions.Experiments are an essential component of the chemistry curriculum, and as a chemistry teacher it is my job to make the laboratory a safe learning environment. In their experiment, the effect of temperature on the equilibrium shift is explored.
Cobalt chloride complex pdf#
[Flinn has a PDF handout and video with a similar lab except acetone is not used. The following year, they concluded that it was worth the wait. After a discussion about the lab, the student decided to take grade 12 chemistry, just to experience it for themselves. In fact, once a grade 11 chemistry student asked about the experiment after eyeing the set of these test tubes in the fume hood where I was evaporating off the acetone. I really enjoy experiencing students' surprised reactions when the two colourful layers form. This lab comes from Chemistry: A Modern Course, Teacher Resource Book, Merrill (1987) - still enlightening students 30 years later. The difference in density is what allows the colour change to occur at the top of the test tube. The acetone undergoes hydrogen bonding with the H 2O, causing the equilibrium to shift to the right producing more of the blue CoCl 4 2- ion. The blue layer on top of a pink layer in the cover photo is accomplished by SLOWLY adding 10 mL of acetone toĥ mL of aqueous 0.2 M CoCl 2. Students experiment with shifting the equilibrium and observing the colour change by
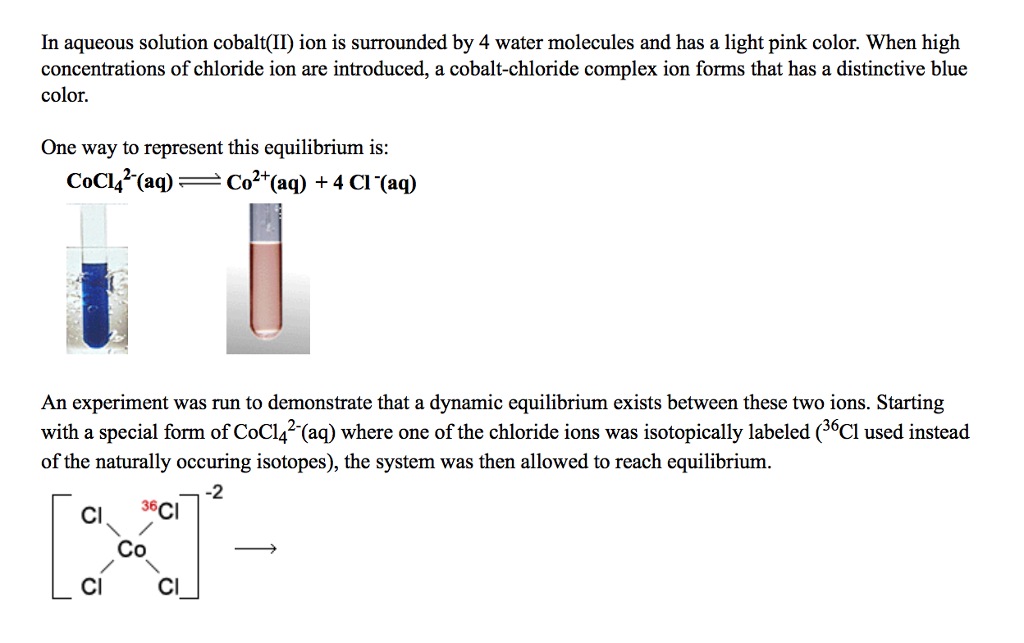
This classic Le Châtelier’s Principle lab explores the reversible chemical reaction:Ĭo(H 2O) 6 2+ + 4Cl – + heat -> CoCl 4 2– + 6H 2O

The blue colour is the result of cobalt chloride complex ions (CoCl 4 2–) in less dense acetone. On the front cover, the pink colour in the test tube comes from cobalt(II) ions in water, Co(H 2O) 6 2+.


 0 kommentar(er)
0 kommentar(er)
